Easy Way to Remember the Carbon Cycle
Click for the student learning guide that accompanies this module.
1. Introduction: Ecosystems and Biogeochemical Cycles
In previous tutorials, we've seen how photosynthesis moves carbon from a gaseous form in the air (carbon dioxide) to a solid form in carbohydrates. During cellular respiration, the carbon in carbohydrate is combined with oxygen to become, once again, carbon dioxide. This exchange of carbon between photosynthesis and respiration is the essence of the carbon cycle, and it's been going on for billions of years.
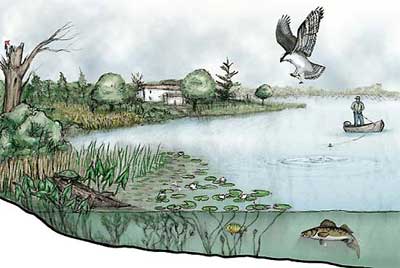
The cycling of elements like carbon is a key feature of any ecosystem. An ecosystem consists of the living community of organisms in an area, plus the non-living components of the environment (the air, the soil, the water, and so on). The living parts (or formerly living parts) of an ecosystem are called biotic components. The non-living parts are referred to as abiotic components.
The diagram on your right shows a lakeside ecosystem. Study it for a moment, and then classify the following parts of this ecosystem as either biotic or abiotic.
The movement of carbon from an abiotic form in the air, to living matter, and then back to the air is an example of a biogeochemical cycle. Let's take this word apart:
- The prefix "bio" relates to life.
- "Geo" relates to the Earth.
- "Chemical" refers to the elements or compounds that we're concerned with.
As an example of a biogeochemical cycle, let's study the carbon cycle.
2. Vocabulary Check
3. The Basic Carbon Cycle: Producers, Consumers, Respiration, and Photosynthesis
Carbon exists in a gaseous form as carbon dioxide in the atmosphere. Through photosynthesis, carbon dioxide gets pulled into plants, combined with water, and transformed into carbohydrate. This process is called carbon fixation. In the diagram below, find the arrow on the upper right that says "photosynthesis" and you'll see carbon being transformed from a gas (carbon dioxide) to solid carbohydrate (the leaves, wood, and other parts of the tree).
.jpg)
Plants are known as producers because they're the organisms that produce carbohydrate, the chemical energy that fuels both plants and animals. Once plants make carbohydrates, a few things can happen. One is that plants can use this chemical energy for their own purposes. Plants, in other words, will perform cellular respiration. As they do, they'll convert carbohydrate back into carbon dioxide and water. This will release carbon dioxide back into the atmosphere.
Another thing that can happen is that animals can consume the carbohydrate made by plants—which is why animals are ecological consumers. When they do, they'll perform cellular respiration, returning carbon dioxide back to the atmosphere. Before going further, make sure that you can identify these flows of carbon in the diagram below.
4. Death and decomposition are also part of the carbon cycle
When plants and animals die, their bodies will be broken down by fungi and bacteria that act as decomposers. Decomposition also involves cellular respiration, meaning that the carbon in the bodies of dead plants and animals gets returned to the atmosphere as carbon dioxide.
Add these factors into the carbon cycle diagram below.
5. Fossil fuel formation, extraction, and combustion add more complexity to the carbon cycle
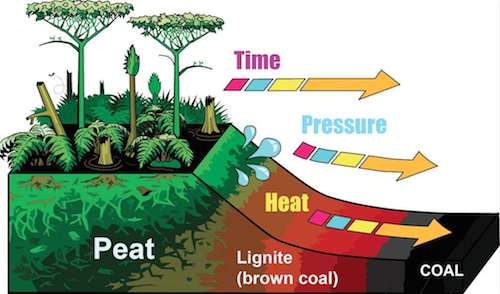
During the Earth's long history, there have been a few periods when forests and algae grew at such a rate that decomposition couldn't keep up with the accumulation of dead organic matter on the floor of forests or the sandy bottoms of oceans.
One of these period was the Carboniferous era, which occurred between 350 and 300 million years ago. During the Carboniferous, all of that carbohydrate accumulated into vast layers. The heat and pressure generated by the weight of these layers transformed this carbohydrate into fossil fuels: coal, petroleum (fuel oil), and natural gas. The diagram above and to the right focuses on coal, but the process is similar for all fossil fuels.

Though these fossil fuels were formed millions of years ago, they mostly lay undisturbed under the ground until the 1800s, when the Industrial Revolution began. Now, fossil fuels are our biggest energy source. Their extraction and processing are billion dollar industries. And combustion is now one of the biggest sources of carbon dioxide in the atmosphere.
As a chemical equation, combustion looks almost identical to respiration. In a car, for example, this combustion reaction occurs.
gasoline + oxygen → energy (heat) + carbon dioxide + water
This human-caused release of carbon is causing the amount of carbon dioxide in the atmosphere to rise. While carbon dioxide levels in the atmosphere were at about 280 parts per million before the industrial revolution, they recently surpassed 400 parts per million. Because carbon dioxide is a greenhouse gas, this is trapping heat in our atmosphere, increasing global temperatures.
Add the formation, extraction/processing, and combustion of fossil fuels to the carbon cycle diagram below.
6. Carbon Cycle: Music Video and Interactive Lyrics
That's the Carbon Cycle. There are additional parts to the cycle: for example, carbon can be trapped in rocks, and then released in volcanoes after hundreds of millions of years. But that goes beyond our goals for right now. I've taken most of what you've read above, and put it into musical form in my Carbon Cycle song. If you're listening to this in a classroom, please listen with headphones so you don't disturb the students next to you.
7. Carbon Cycle: Cumulative quiz
When you're confident that you understand the material above, take the quiz below.
Links
-
- Continue on to the next tutorial in the series, the Nitrogen Cycle.
Source: https://learn-biology.com/carbon-cycle-tutorial-and-quiz/
0 Response to "Easy Way to Remember the Carbon Cycle"
Post a Comment